Pelvic Mass Assessment
Roughly 20% of women will develop a pelvic mass in their lifetimes. Imaging, single analyte serum testing, and physical exam are common methods to assess pelvic masses for malignancy. However, multiple studies have shown that these individual modalities can miss many cancers, as the sensitivity of these tests is typically low for early stage cancers, premenopausal patients and certain ovarian cancer subtypes.
Effectiveness of Common Modalities
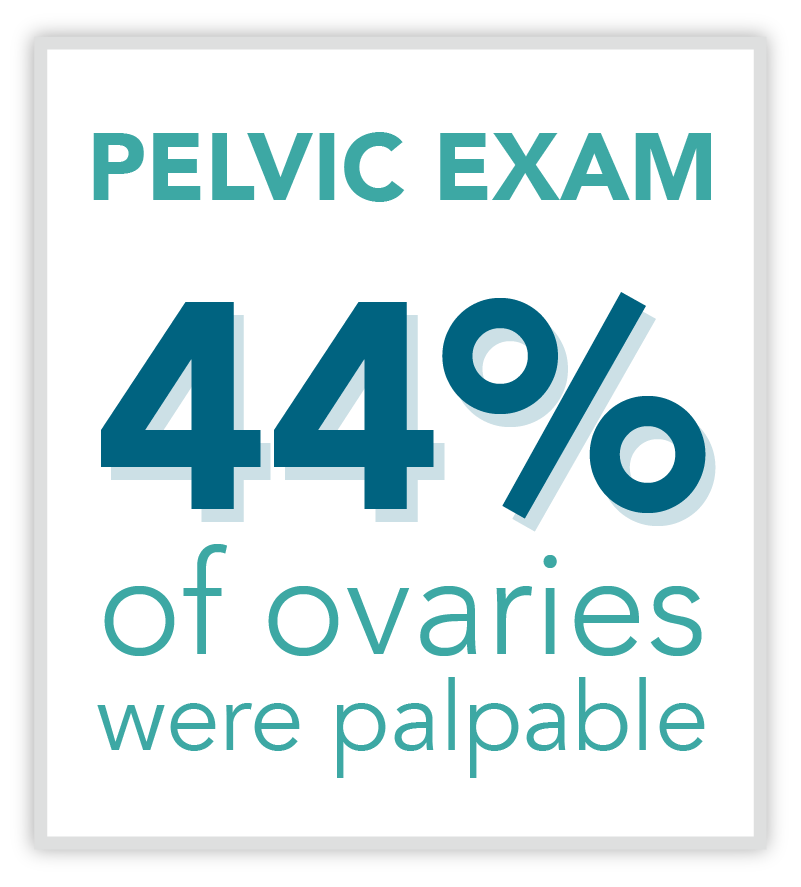
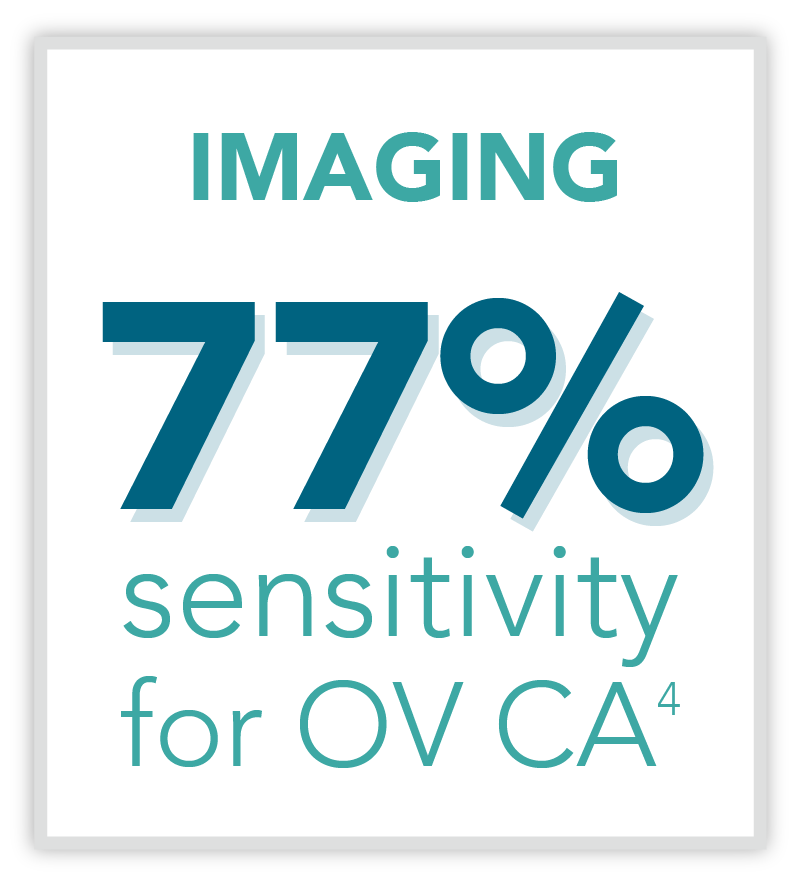
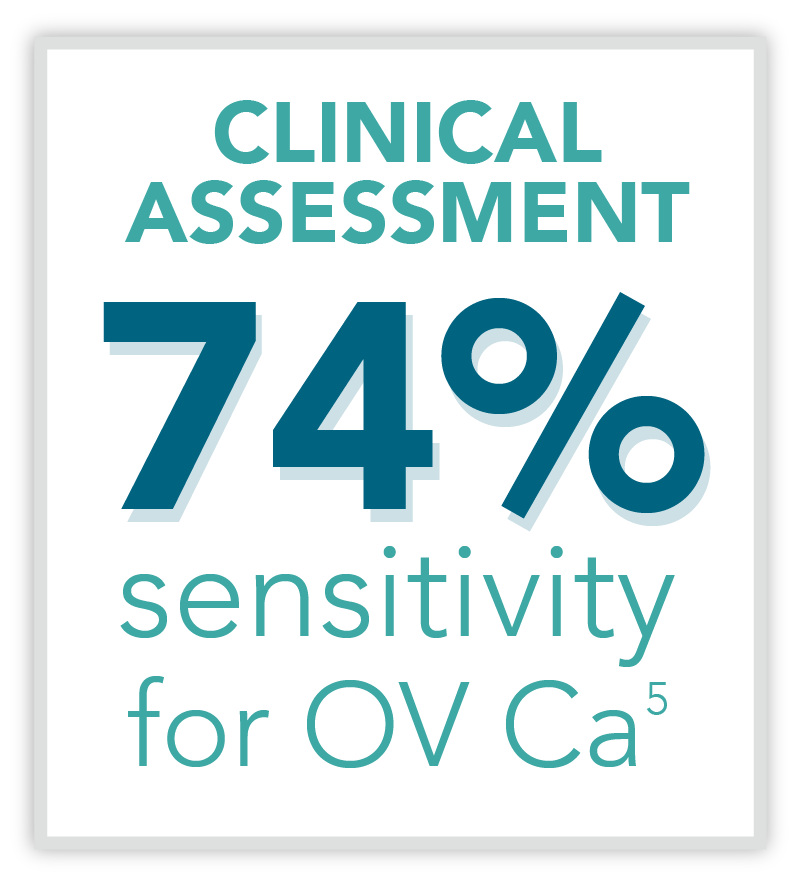
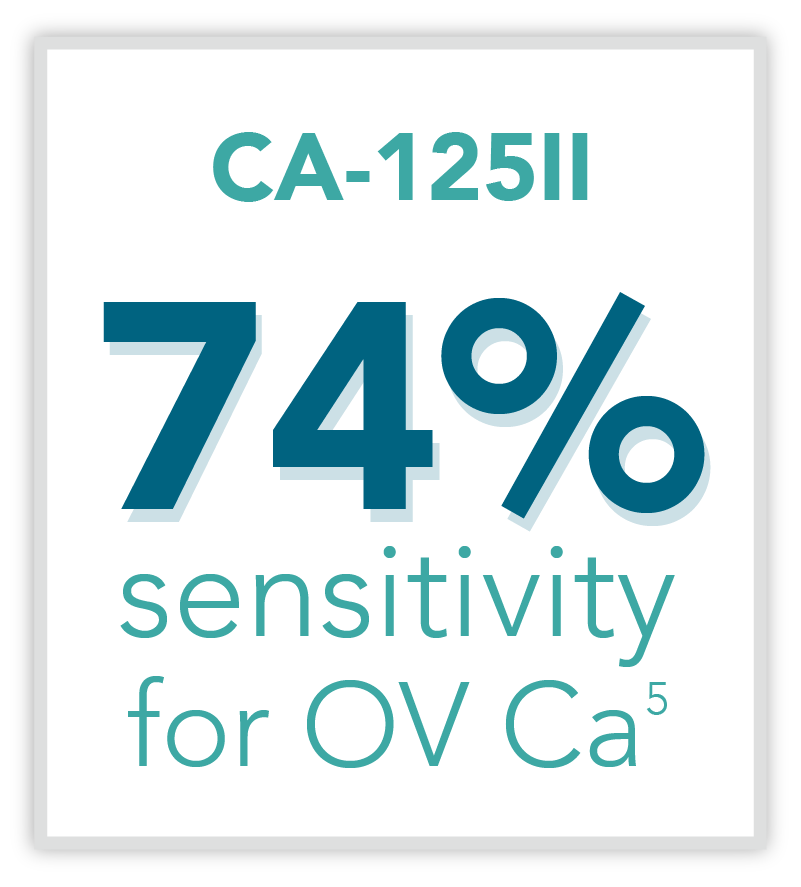
The Status Quo
Traditionally, imaging and clinical assessment are the most relied-upon methods of assessing pelvic masses for malignancy. This routine method of identifying ovarian cancer has limitations due to the following reasons:
- Indeterminate findings in imaging6
- Interobserver variation in imaging7
- Variability in patient disclosure of ovarian cancer risk factors
- Low sensitivity for early stage cancers and in premenopausal patients8,9
Serum Testing
CA-125II is commonly thought of as a tool for risk assessment as elevated levels may coincide with the presence of advanced ovarian cancer. However, CA-125II alone is not cleared for use to evaluate ovarian masses prior to surgery. Up to 50% of early stage cancers are not detected by the cancer antigen marker. Rather, the FDA cleared CA-125II for monitoring patients who already have ovarian cancer, to facilitate treatment and cancer surveillance. Multiple studies have shown that CA-125II is currently ordered inappropriately, with only one-third of the tests used as intended.10,11
Many common singly analytes are not intended by the FDA to detect a broad range of ovarian cancers. These include:
| Analyte(s) | Clinical Use | Cancer Type |
|---|---|---|
| CA-125II | Monitoring disease progression, response to therapy | Ovarian |
| HE4 | Monitoring recurrence or progression of disease | Ovarian |
| AFP, LDH, hCG | Aid in diagnosis, management and prognosis | Ovarian (germ cell) |
| CA19-9 | Monitoring disease status | Pancreatic |
| CEA | Aid in management and prognosis | Not specified (commonly for colorectal) |
- Carney ME, et al., Gynecol Oncol. 2002 Jan;84(1):36-42.
- Earle CC, et al., J Natl Cancer Inst. 2006 Feb 1;98(3):172-80
- Ueland, FR et al., Gynecol Oncol. 2005 Nov;99(2):400-3
- Goodrich ST, et al., Am J Obstet Gynecol. 2014 Jul;211(1):65.e1-65.e11
- Bristow RE, et al., Gynecol Oncol. 2013;128:252-259
- Timmerman D, et al., Ultrasound Obstet Gynecol 1999;13:11–16
- Levine D, et al., Ultrasound Q. 2010 Sep;26(3):121-31.
- Ueland FR, et al., Obstet Gynecol. 2011;117(6):1289-1297
- Longoria TC, et al., Am J Obstet Gynecol. 2014 Jan;210(1):78.e1-9
- Moss EL, et al., J Clin Pathol. 2005 Mar; 58(3): 308–312.
- Petignat P, et al., Eur J Cancer. 2000 Oct;36(15):1933-7.
- Bristow RE, et al., Am J Obstet Gynecol. 2013 Dec;209(6):581.e1-8