OVA1 vs. CA-125
There are a limited number of ovarian cancer risk assessment tests available today. Below is information on how OVA1 compares to and outperforms the other available tests for women with adnexal masses.
What is the difference between OVA1 and CA-125?
OVA1 (MIA), unlike CA-125, is cleared by FDA for the pre-surgical assessment of ovarian cancer risk. OVA1 measures a panel of 5 biomarkers—including CA-125—which sensitively detects malignancy across all stages and subtypes of ovarian cancer. As a result, fewer cancers are missed than CA-125 or other common methods. Because of the risk of ovarian cancer spreading to other areas, the high sensitivity of OVA1 (MIA) in detecting a malignant risk becomes extremely important.
What is CA-125?
CA-125 (cancer antigen 125) is a protein found in the blood and is frequently used as a biomarker for detecting the presence of ovarian cancer. It is elevated in certain types of ovarian cancer, but not others. A CA-125 test can be used to determine if a patient’s cancer has recurred and in some instances, to assess the risk of ovarian cancer before diagnosis.
Because the presence of ovarian cancer may coincide with elevated levels of CA-125, it has also been used historically to assess risk of malignancy before surgery, even though such use is not approved by the FDA. The test is not completely accurate as many noncancerous conditions can also result in increased levels of the protein. As a result, up to 50% of early stage cancers are not detected by CA-125.
Early Stage Ovarian Cancer Detection by Sensitivity
| Product | Early Stage (Stage I and II) | Pre-menopausal Early Stage | Post-menopausal Early Stage |
|---|---|---|---|
| CA-125 | 66% | 46% | 75% |
| OVA1* | 91% | 91% | 92% |

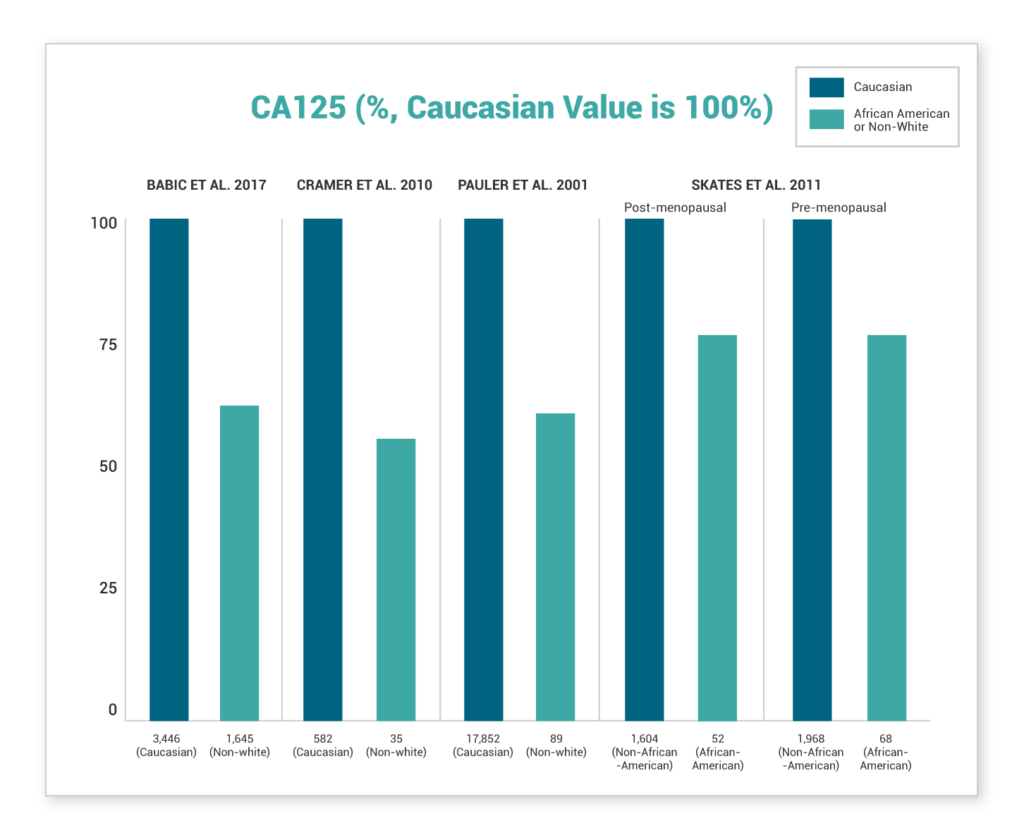
Several recent studies show lower values of CA-125 were demonstrated in African-American women or non-white women
Non-White Women, and African-American Women in Particular, Display Significantly Lower CA125 Values Compared to Caucasian Women. This disparity is found in healthy women, women at high risk for ovarian cancer, and women with ovarian cancer(7-10).
What is the difference between OVA1 and ROMA?1,2,3
ROMA and OVA1 (MIA) have the same FDA intended use. Both tests are used to assess the cancer risk of adnexal masses prior to surgery and must be interpreted in conjunction with an independent clinical and radiological assessment and are not intended as a screening or stand-alone diagnostic assay. However, the performance of the two tests is different.
This can be demonstrated in a side-by-side view of individual validation studies and third-party head-to-head comparisons.
| Product | Ov Ca Subtypes | (n) | Prevalence | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| ROMA (Moore et al. 2011) | All ovarian cancers | 472 | 18.9% | 80.9% | 74.9% | 42.9% | 94.4% |
| OVA1 (Bristow et al. 2013) | All ovarian cancers | 494 | 18.6% | 92.4% | 53.5% | 31.3% | 96.8% |
OVA1 (MIA) has robust data of early stage sensitivity with over 86 cancers evaluated.
Moore et al., 2011 validation study
| ROMA | Sensitivity | (n) |
|---|---|---|
| Stage 1 | n/a | – |
| Stage 2 | n/a | – |
| Early Stage | 75% | 12 |
Longoria et al., 2013
| OVA1** | Sensitivity | (n) |
|---|---|---|
| Stage 1 | 89% | 61 |
| Stage 2 | 100% | 25 |
| Early Stage | 92% | 86 |
OVA1 (MIA) has strong evidence of high sensitivity across a broad range of subtypes. This is clearly characterized in non-epithelial ovarian cancers, LMPs, metastases and other malignancies.
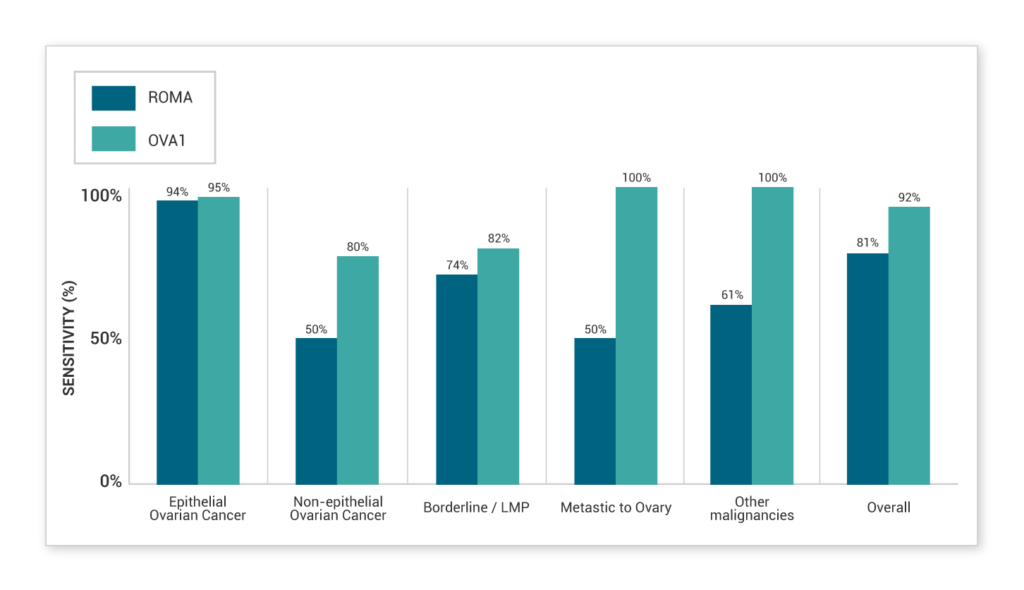
Head-to-head comparison study4
Grenache et al. 2015 evaluated both OVA1 (MIA) and ROMA in all samples which included 31 surgically confirmed malignancies in 146 adnexal masses. The results showed that OVA1 (MIA) had higher sensitivity, which allows optimal distribution of cancers to specialists and confidence in managing negative tests as they were more likely benign cases.
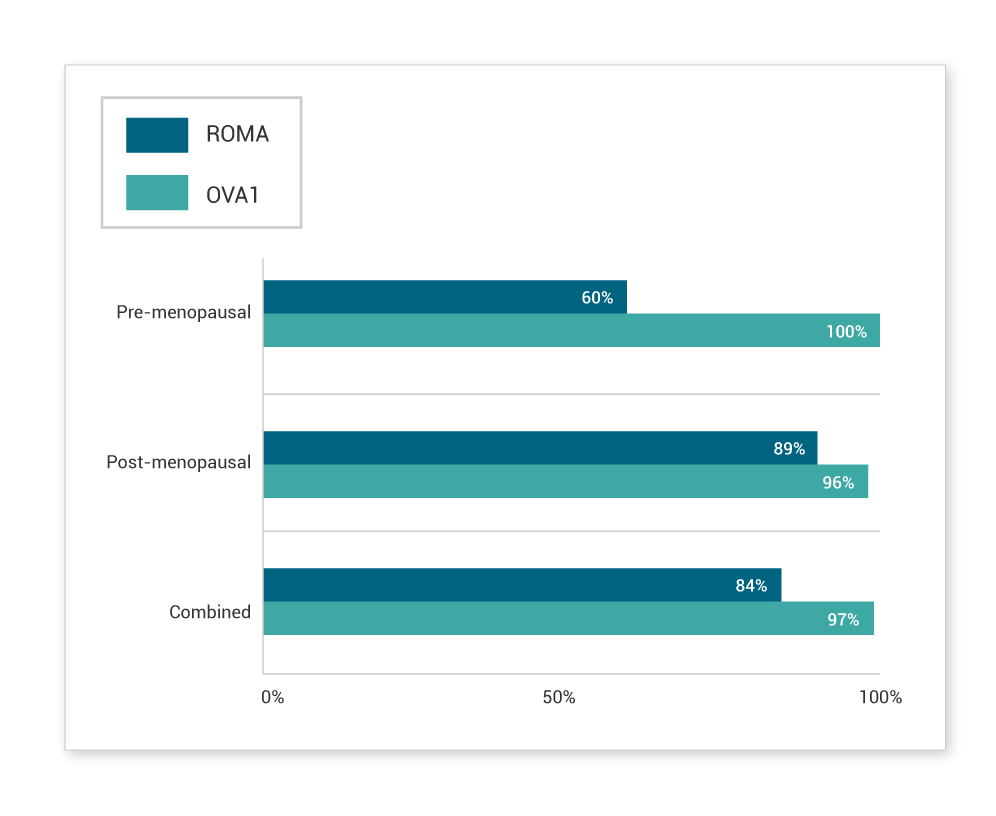
Characterization of ROMA5
In Lennox et al.’s independent study, ROMA performed well for advanced ovarian cancer and high-grade serous histology by capturing 93% and 94% of these subtypes, respectively. However, a high false-negative rate was observed for early-stage cancers, pre-menopausal patients and other histological subtypes.
| ROMA Cancers Missed | Sample Size | (n) |
|---|---|---|
| Advanced Ovarian Cancer | 7% | 54 |
| High-grade Serous Subtype | 6% | 58 |
| Stage 1 | 46% | 35 |
| Pre-menopausal Status | 27% | 30 |
| Endometrioid Subtype | 35% | 18 |
| Clear Cell Subtype | 30% | 11 |
| Overall | 21% | 104 |
- Moore RG et al., Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
- Bristow RE, et al., Gynecol Oncol. 2013;128:252-259
- Longoria TC, et al., Am J Obstet Gynecol. 2014 Jan;210(1):78.e1-9
- Grenache DG, et al., Clin Chim Acta. 2015 Jan 1;438:358-63.
- Lennox GK et al., Int J Gynecol Cancer. 2015 Jun;25(5):809-14.
- American Congress of Obstetricians and Gynecologists, Practice Bulletin 174; 2016 Nov
- Pauler, D., et al. Factors Influencing Serum CA125II Levels in Healthy Postmenopausal Women. Cancer Epidemiology, Biomarkers & Prevention, 10: 489-493, 2001.
- Skates, S., at al. Large Prospective Study of Ovarian Cancer Screening in High-risk Women: CA125 Cut-point Defined by Menopausal Status. Cancer Prevention Research, 4(9), 1401–1408, 2011.
- Cramer, D., et al. Correlates of the pre-operative level of CA125 at presentation of ovarian cancer. Gynecologic Oncology, 119(3), 462–468, 2010.
- Babic, A., at al. Predictors of pretreatment CA125 at ovarian cancer diagnosis: a pooled analysis in the Ovarian Cancer Association Consortium. Cancer Causes & Control : CCC, 28(5), 459–468, 2017.