
Confidently Move From Assessment to Action
A portfolio of blood tests developed to assess the risk of ovarian cancer in any woman with an adnexal mass.
Adnexal masses present a unique diagnostic dilemma. For the first time, healthcare providers have a set of blood tests that – when combined with their clinical experience, ultrasound, and the patient’s history – allow them to confidently develop a medical management plan and include the right specialists at the right time.

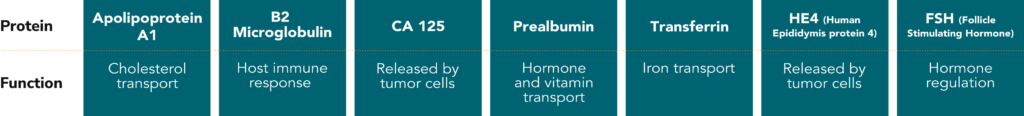
The OvaSuiteSM portfolio of blood tests applies proprietary algorithms incorporating patient features (such as menopausal status) and the levels of up to 7 biomarkers in the blood.
OvaSuite risk assessment tests offer the following to healthcare providers and their patients:
- Enhanced provider confidence in the chosen medical management plan
- Efficient triaging of specialist referrals for only higher risk patients
- Reduced patient anxiety through improved information about malignancy risk
- Avoidance of potentially unnecessary or premature surgeries
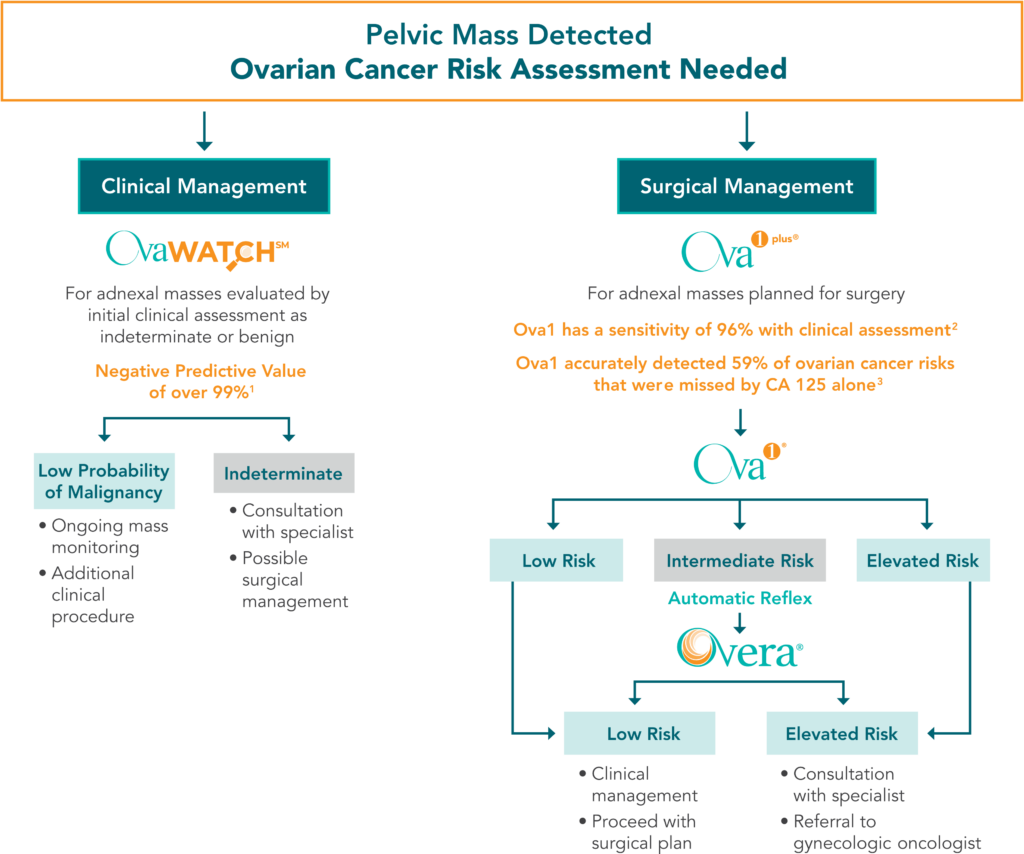
Whether a healthcare provider is performing an initial clinical assessment or planning for surgery, OvaSuite has a tool to help them confidently develop a management plan for all points along the healthcare journey. These results, when combined with the providers judgment, imaging and patient history, provide better clinical insight over the single biomarker tests used in the past.

Learn more about the OvaSuite portfolio of products by listening to our Aspira Women's Health Key Opinion Leader Webinar!
Expert Consultation is available for those who have already ordered an OvaSuite test as part of the Aspira Women’s Health Suite of Services.
Utilize the OvaSuite portfolio of products and confidently move from assessment to action.
A single blood draw – which can be performed at a number of national and regional laboratories or in a healthcare provider's offices using our proprietary testing kit – is all that is needed.
Contact us to learn more, order kits, or to locate one of our phlebotomy partners.
- Analytical Validation of a Deep Neural Network Algorithm for the Detection of Ovarian Cancer. Reilly G, Bullock R, Greenwood J, Ure DR, Stewart E, Davidoff P, DeGrazia J, Fritsche H, Dunton CJ, Bhardwaj N, and Northrop LE. JCO Clinical Cancer Informatics 6:e2100192 2022
- Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecologic Oncology, 128(2), 252-259.
- Dunton, C. J., Hutchcraft, M. L., Bullock, R. G., Northrop, L. E., & Ueland, F. R. (2021). Salvaging detection of early-stage ovarian malignancies when CA 125 is not informative. Diagnostics, 11(8), 1440.


