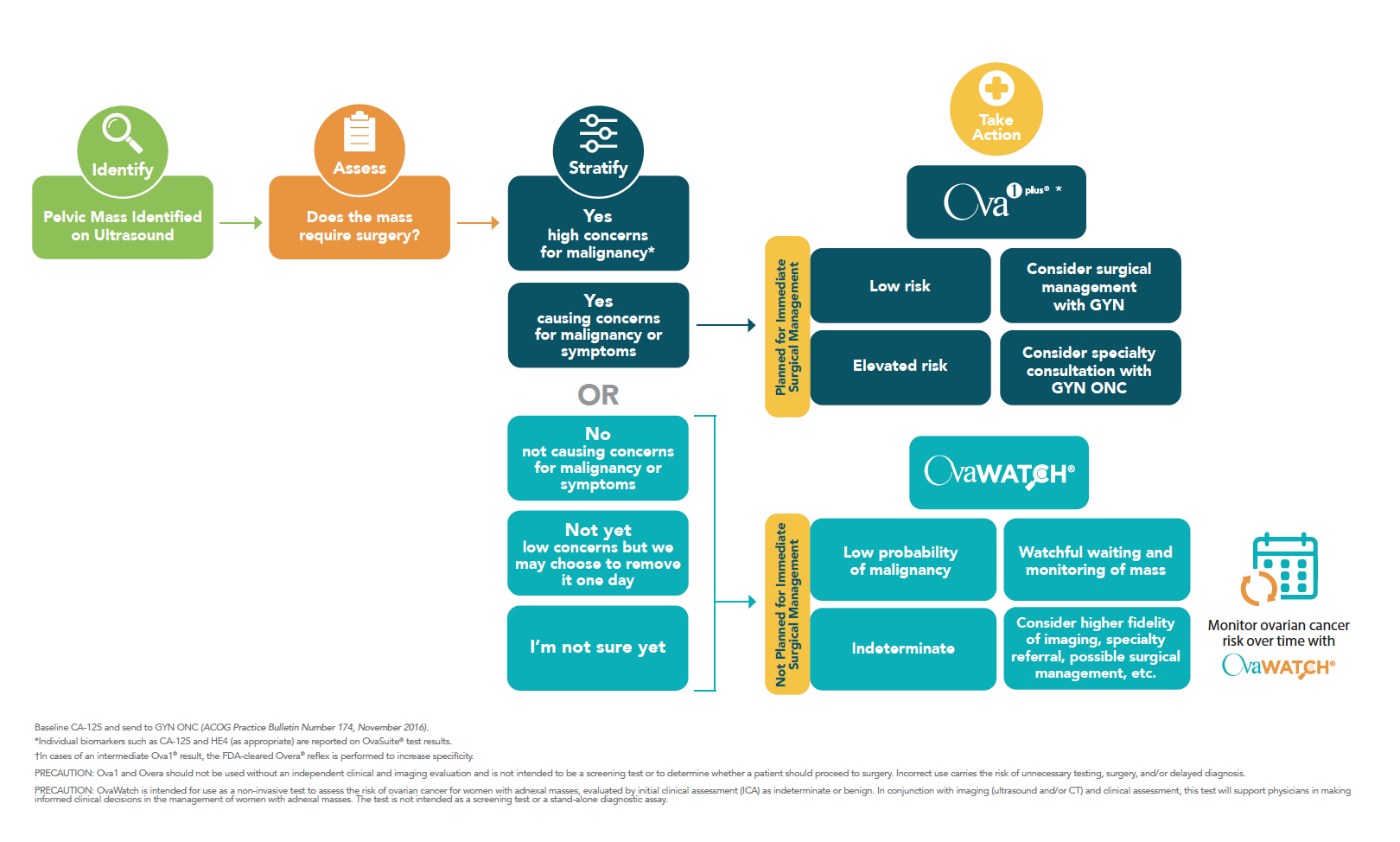
How do I know which product I need?
What is Ova1Plus®?
Ova1Plus is a workflow, which uses Ova1®, a qualitative serum test intended as an aid to further assess the likelihood of malignancy in women with an ovarian adnexal mass for which surgery is planned when the physician’s independent clinical and radiological evaluation does not indicate malignancy, as the primary test and Overa®, a second-generation biomarker test intended to maintain Ova1’s high sensitivity while improving specificity, as a reflex for Ova1 intermediate range results, leveraging the strengths of Ova1’s MIA sensitivity and Overa’s (MIA2G) specificity to reduce incorrectly elevated results.
Do I have to order Overa separately when using Ova1Plus?
No, you do not need to order Overa separately. When you order Ova1Plus, Overa will automatically be performed if the Ova1 result falls into the intermediate-risk category. This workflow is built into Ova1Plus, streamlining the testing and risk assessment process.
Does the workflow to Overa incur extra costs?
No, the workflow of Ova1Plus does not incur additional costs. Ova1Plus is designed to provide a comprehensive risk assessment, including the workflow with Overa if needed, without increasing the overall cost to the provider or patient.
PRECAUTION:
OVAWATCH IS INTENDED FOR USE AS A NON-INVASIVE TEST TO ASSESS THE RISK OF OVARIAN CANCER FOR WOMEN WITH ADNEXAL MASSES, EVALUATED BY INITIAL CLINICAL ASSESSMENT (ICA) AS INDETERMINATE OR BENIGN. IN CONJUNCTION WITH IMAGING (ULTRASOUND AND/OR CT) AND CLINICAL ASSESSMENT, THIS TEST WILL SUPPORT PHYSICIANS IN MAKING INFORMED CLINICAL DECISIONS IN THE MANAGEMENT OF WOMEN WITH ADNEXAL MASSES. THE TEST IS NOT INTENDED AS A SCREENING TEST OR A STAND-ALONE DIAGNOSTIC ASSAY.
OVA1 AND OVERA SHOULD NOT BE USED WITHOUT AN INDEPENDENT CLINICAL AND IMAGING EVALUATION AND IS NOT INTENDED TO BE A SCREENING TEST OR TO DETERMINE WHETHER A PATIENT SHOULD PROCEED TO SURGERY. INCORRECT USE CARRIES THE RISK OF UNNECESSARY TESTING, SURGERY, AND/OR DELAYED DIAGNOSIS.
*In cases of an intermediate Ova1® result, the FDA-cleared Overa® reflex is performed to increase specificity